
There are many species of caridean
shrimps that live in fresh water: in lakes, streams, rivers. Many
of these are completely adapted to the f.w. habitat in that they pass the
entire life cycle in freshwater. The planktonic larval stages of
their marine ancestors are reduced, abbreviated or completely eliminated,
i.e., embryos hatch out as benthic juveniles. However, many other
species have not lost their connection to the sea and, although the adults
live all or most of their lives in freshwater, the larvae are planktonic
and require some salinity for development. They must develop in the
brackish water of estuaries or in coastal seas. After larval development,
the newly settled benthic juveniles must migrate back up streams and rivers
to the adult habitat. Some of these juvenile migrations have been
observed and studied in recent years and can be quite spectacular.
This life cycle is termed "amphidromous" and is exhibited by many species
in the families Atyidae, Palaemonidae (esp. the genus Macrobrachium) and Xiphocarididae. See the review of amphidromy in shrimps in Bauer (2013) and in Chap. 9 of the Bauer (2023) book on shrimp biology.
 |
 |
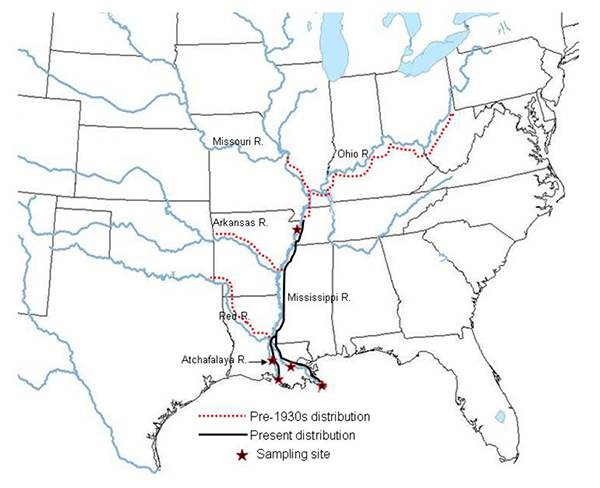
| M. ohione, with typically small male (left) and larger female (right) | Past and present d istribution of M. ohione in the Mississippi River system: |
M. ohione is one of several species of Macrobrachium that inhabit coastal river systems emptying into the Gulf of Mexico and the S.E. Atlantic coast of the U.S.A. All species were formerly more abundant but human impacts on rivers (especially dams and other river control measures) have considerably reduced populations. Until about the mid-1930's, M. ohione was so abundant in the Mississippi and lower Ohio river systems that it supported a small but significant fishery for both human consumption and for sale as bait. It is the still the favorite bait item for commercial fishermen in the lower part of this river system. The shrimp must have served as a major prey item for river fishes, and its decline in the northern part of its range may have affected fish productivity. It is still fairly abundant in the lower Mississippi and Atchafalaya rivers in Louisiana, where we studied it.
M. ohione females must either release (hatch) embryos
upstream, with the first-stage (non-feeding) larvae drifting down to the sea OR the females
may simply make the trip themselves, migrating downstream to estuaries
to hatch the embryos for larval development in the sea. We studied
both the female downstream migration as well as the "return" upstream juvenile
migration from the sea after larval development. This research is significant
not only for understanding the evolution of amphidromous life cycles but
also to determine how human impacts on these migrations may have caused
the decline of this ecologically and commercially important river species.
If human impacts are responsible, our work may contribute to the conservation
of the species where it is still abudant and to its restoration in northern
parts of its range where it was once so important. Our work was supported
by the NOAA Louisiana Sea Grant College Program, which we gratefully acknowledge
(grant No. NA06OAR4170022, Project No. R/SA-04 to RTB and Louisiana State
University and the University of Louisiana, Lafayette. Statements, findings,
conclusions, and recommendations are those of the authors and do not necessarily
reflect the views of Louisiana Sea Grant or the U.S. Department of Commerce).
Larval Biology
We studied larval requirements to answer the questions: how long can hatching (stage 1) larvae drift downstream in fresh water before encountering the saltwater necessary for further development? What is the optimal salinity for the critical stage 1 to molt to the first feeding stage-2 molt? This helped us to determine whether females must migrate all the way to the correct salinity in estuaries to hatch larvae and/or whether they can simply migrate to within a larval "drifting distance" upriver to release the stage-1 larvae.
STAGE-1 to STAGE-2 MOLT IS CRITICAL
|
 |
| Stage-1 larva: Does NOT feed (relies on yolk droplets left over from the embryo) | Stage-2 larva: Begins to feed and go on to further larval stages |
Previous work has shown that Stage-1 larvae maintained in fresh water begin to die after 5 days; those maintained in partial seawater after hatching (15 ppt) are able to molt to stag- 2 within a few days and thus begin feeding and continuet larval development (Bauer and Delahoussaye, 2008).
How long can stage 1-larvae drift in freshwater before arriving in saltwater and what is the optimal salinity needed for the stage-1 to stage 2 molt? The coastal estuaries (e.g., Atchafalaya Bay) of Louisiana have very low salinities during the breeding season.
ULL undergraduate Nick Romer and collaborators, with funding from the LA Sea Grant Undergraduate Research Opportunities Program (UROP), perfomed an experiment, using a factorial design, to determine the optimal drifting (exposure) time in fresh water and optimal salinity for the critical stage-1 to stage-2 larval molt (Rome et al., 2009). Hatching larvae were obtained by collecting females from the field and were maintained on laboratory water tables until hatching. Alternately, females with maturing ovaries are brought in to the lab, maintained with some of the males. When such females with mature prespawning ovaries molt, mating occurs, and embryos are attached beneath the female for incubation and later hatching (stage-1 larvae shown below, at right). Females near hatching are isolated in hatching tanks and stage 1 larvae are collected for the experiments. In the experiments, larvae were maintained in fresh water in treatments which vary in the number of days in freshwater prior to introduction into seawater of differing salinities.
 |
 |
 |
 |
Females (with embyros) maintained individually in buckets |
Hatching bucket with aeration |
Female in hatching bucket |
Macrobrachium ohione stage 1 larvae hatched in the laboratory |
 |
 |
 |
Replicates of different larval treatments in incubator |
Culture dishes with larvae and aeration |
Very slow aeration (~1 air bubble per second) |
The results of the experiment showed that larvae kept in fresh water for 1 or 3 days before a change to saline water at 6 or 10 ppt showed a greater frequency of molting than those kept for longer (> 5 days) in fresh water or changed to less saline water (2 ppt). Non-molting larvae died or were moribund within 11 days of hatching. In summary, larvae need to reach estuarine water with >5 ppt salinity within a few days in order to molt, feed and complete larval development
Downstream Migration of Stage 1 Larvae and Reproductive Females
Another aspect of our work was to sample larvae and reproductive-sized females throughout the year at different sites upriver in the Atchafalaya and Mississippi rivers in Louisiana to determine (1) if only stage 1 larvae drifted downstream to the Gulf, or did molting to later larval stages occur before reaching saline water? and (2) do females carrying embryos increase in abundance (migrate downstream) in the lower parts of the Atchafalaya and Mississippi Rivers during the reproductive season?
As suggested by the experiments described above, we ONLY found stage 1 larvae in our stations up to 150 km from the Gulf and even within the Atchafalaya Delta estuary. In the spring reproductive season, the river flow is highest and sweeps water out of Atchafalaya Bay, presumably taking Stage-1 larvae with it. Therefore, the molt to Stage-2 (first feeding stage) and subsequent larval stages must take place in saline waters out in the open Gulf.
We also found that the distribution and abundance of females with embryos near to hatching was much greater downstream than further upstream during the reproductive season, indicating that gravid females were indeed migrating down to the Gulf to hatch their larvae as close to downstream estuaries rather than upstream. The subadult/adult population was readily sampled with baited river shrimp traps, shown below. We were helped in this sampling by the Entergy River Bend Power Plant on the Mississippi and in the Atchafalaya Delta and Mississippi (Pass-a-Loutre) estuaries by the Louisiana Dept. Wildlife and Fisheries. In Bauer and Delahoussaye (2008), we reported that the relative abundance of females with embryos near hatching is far higher near or in the Atchafalaya Bay estuary than at Butte La Rose, 150 km upstream in the spring, indicating a movement (hatching migration) of reproductive females to the downstream estuary. In the Sea Grant project, we extended these observations to the Mississippi River populations with similar results (Olivier and Bauer, 2011).
 |
 |
 |
 |
| Tyler Olivier and Jim Delahoussaye sampling plankton (shrimp larvae) in the Atchafalaya Rivr near Butte La Rose, ~150 km upstream of the Atchafalaya Delta Estuary | Ray Bauer attaching end bucket of plankton net |
Jim Delahoussaye, Louisiana naturalist and collaborator in the project with river shrimp trap (funnel oriented downstream | Trap with good catch of M. ohione |
Upstream Migration of Juveniles After Larval Development
If larval development takes place
in estuaries or the open sea, the resulting small juveniles need to make
a migration back upriver to the adult f.w. habitat. Juvenile migrations,
in the form of long lines of juveniles crawling or swimming upriver along
the shore, have been more and more studied in the last few decades (e.g., various investigators in Japan, Alan Covich and colleagues, Jeff Holmquist in Puerto Rico; Peter Novak in Australia,Watcharapong ("Win") Hongjamrassilp in Thailand
). We made a rough calculation on when such a migration might
occur based on spawing season (April-July), as well as estimates of larval development
time from other Macrobrachium spp., the distance from the Atchafalaya
Delta etc. We started looking for juveniles in midsummer; we observed the very spectacular migration for several years, beginning
in mid to late July and terminating in September. Juveniles
swim right along the bank, only at night, and often at very high densities.
We have studied detailed characteristics of this migration: marine
origin of the juveniles using stable istope ratios (with Brian Fry at LSU),
growth during migration, and swimming speeds. Tyler Olivieer, a former ULL doctoral student, now Dr. Olivieer, made observations on fast and how far juveniles swim during the night. With this information,
we tested hypotheses about the present and past distribution of this
species, as well as suggested strategies for the conservation and restoration
of this species, in which human impacts on the juvenile migration are a
probable cause of the population collapse in most parts of its former range.
 |
 |
| Small (~1 cm long) M. ohione juveniles migrating upstream along the bank of the Atchafalaya River | Close up of juenile migrators swimming upstream (from right to left in this photo) along the river bank |
Where to go from here: There are a variety of important questions to be asked and answered, here are a few (see Bauer and Delahoussaye, 2008; Bauer 2013 review on amphidromy in shrimps):
1. Do females far upstream make the migrations down to the Gulf? Up to the mid 1940's, populations were abundant over 1000 km upstream in the upper Mississippi and lower Ohio Rivers,
2. Likewise, can the juveniles migrating back upstream from the Gulf of Mexico reach as far north as the former far upstream populations indicate? or did larval development somehow upstream in fresh water? In localized streams flowing over ancient salt deposits ("salines") ??? (discussed in Bauer and Delahoussaye, 2008; Bauer, 2013).
3. There is preliminary (unpublished) evidence from sampling of juveniles as far north as mid-Arkansas in the Mississippi that juveniles do migrate at least that far north. These juveniles are much larger than those occurring in migrations near the Gulf, indicating that the juveniles are molting and growing as they migrate north. If they settle far up north and become reproductively active, do they or their stage-1 larvae reach the Gulf to complete development? If not, those far upstream populations are (were) reproductively dead ("sink" populations whose "source" is the Gulf of Mexico).
4. Where do the early-stage larvae go after entering the Gulf of Mexico waters to complete development, and how do the post larvae find the mouth of a river or stream into begin the upstream juvenile migration to their freshwater adult habitat?