Latitudinal Variation in Breeding Patterns
One goal of biological studies is to find generalizations
about organisms that can be used to explain patterns observed in nature.
These generalizations also help to predict how organisms will respond under
particular environmental situations, present and future. Part of my work
on reproduction in shrimps has been to describe the temporal patterns of
breeding in hopes of determining the causal factors of such patterns. Especially,
some of my studies have examined the seasonality of reproduction: how does reproductive (breeding) vary seasonally with latitude in shrimps? Early workers such as J.H. Orton (Orton, J.H. 1920. Sea temperatures, breeding and distribution of marine animals. J. Mar. Biol. Assoc. U.K. 12: 339-366.) observed that breeding seasons of marine animals were continuous throughout the year. He attributed this to what he considered a benign and stable throughout the year in shallow tropical seas, with particular emphasis on water temperature, which has an important influence on basic metabolic processes, including reproduction, which is speeded up with higher temperatures and slowed down or inhibited by colder ones. Thus, according to this hypothesis, reproduction should be continuous in the warm tropical seas and increasingly seasonal (restricted) at higher latitudes. Gunnar Thorson (Thorson, G. 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews of the Cambridge Philosophical Society. 25: 1-45) proposed that the availability of food supply in the plankton the important causal factor for marine invertebrates, a majority of which produce planktonic larvae. Plankton productivity is dependents on various environmental factors, including temperature but also nutrient supply and photoperiod, all of which vary seasonally with latitude. Of course, it must be remembered that this simple paradigm can be modified by localized currents, upwelling and river runoff.
Does reproductive pattern in shrimp species vary with latitudinal trends in environmental variables such as temperature, nutrient supply and plankton productivity or other factors? Comparisons among species from different areas and latitudes of the world is a good way to test for latitudinal trends in reproductive and related life-history traits A useful approach for answering this question is to make comparisons and contrasts among unrelated species from similar latitudes and habitats: environmental factors are held constant, but the phylogenetic component of reproduction varies. On the other hand, related species from different latitudes but similar habitats can be compared. In this case, their ancestral reproductive biology (phylogeny) is similar; differences in reproductive pattern might then be due to latitudinal differences in environmental factors which can then be investigated individually (Bauer, 1992a, 2023).
I tested this approach using seagrass shrimps of West Atlantic and Caribbean species, with both several caridean and penaeoid shrimps such as Sicyonia spp. . As a young assistant professor at the University of Puerto Rico, Rio Piedras (San Juan), I had the opportunity to sample shrimps in extensive seagrass meadows, in particular, those at Dorado on the north coast. My sponsor during my postdoctoral fellowship at the National Museum of Natural History (Smithsonian), Ray Manning, had told me these tropical seagrass beds were rich with diverse shrimp species, and advised me to sample at night, when shrimps were most active. This turned out to be great advice.
I set out to take monthly samples, both during day and night, for a year and make various measurements on reproductive activity. In the 9 most abundnt caridean species (1985a,b), this included relative frequency of ovigerous females (those incubating embryos beneath the abdomen) and degree of ovarian maturation. In the most abundant penaeoid species, Sicyonia laevigata and S. parri, a master's student (Luis Rivera) and I used degree of ovarian maturation, as penaeoids do not incubate embryos. The seagrass meadows were sampled for shrimps using pushnets (another Ray Manning piece of advice) which are quite good at collecting good samples of seagrass shrimps (total of 75,000 carideans and 7,500 penaeoids over 12 months). The samples included a tremendous diversity of other marine animals, especially gastropods (snails) and other crustaceans. All these species were sorted out and identified, a tremenous job. Fortunately, I was able to recruit a group of undergraduate assistants to help both with sampling and sorting, to whom I am eternally grateful.
 |
 |
| Seagrass meadow flora, north coast of Puerto Rico, consisting primarily of Thalassisa testudinum (broad leaves) and Syringodium filiforme (narrow tubular leaves); note crust of microalgae on Thalassia leaves; this is good food for some of the shrimp species. |
Pushnet used in collecting samples of seagrass shrimps in a Thalassia meadow |
 |
 |
| Caridean Thor manningi, Puerto Rico; note color camouflage of shrimp which include the green color of seagras and that of the microalgae encrusting the seagrass blade |
Sicyonia laevigata, a penaeoid species, semiburrowed in sand; this and S. parri were the most abundant species of penaeoids, along with several Metapenaeopis species |
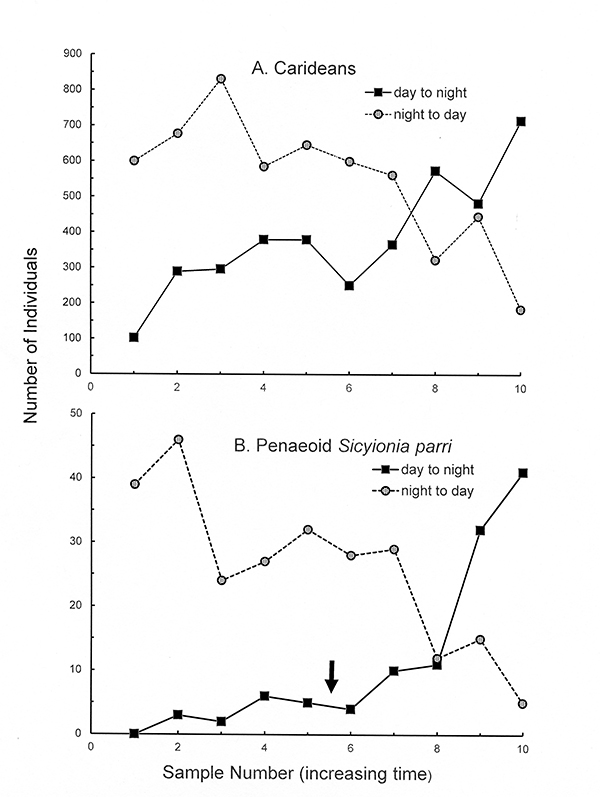
Sampling during the day and then later at night demonstrated that shrimps and hermit crabs are very nocturnal in their activity (see Bauer, 1985 a-c). Most shrimp species are more abundant (collectable) at night because of their activity (feeding, mate search etc.) during that time. During daylight hours, some species burrow down into the bottom while "blade" species cling more tightly to seagrass blades, making them less available to collection by nets. During the day, hermit crabs are found clustered in great numbers around pieces of coral debris or other structures on the seagrass meadows. At night, they leave these clusters and actively spread out over the meadows, engaging in as yet unknown activities (one can hypothesize feeding, search for mates, shells etc.) Nocturnal activity has evolved in these small seagrass crustaceans to reduce detection by day-active fish predators which hunt by vision.
|
Day-night comparisons in collectability (activity) of seagrass shrimps in Puerto Rico. Day to night: samples began just before sunset and terminated during the night. Night to day: samples began just before sunrise and terminated in early daylight hours.(A) Total caridean species. (B) Penaeoid Sicyonia parri. In (B), the day to night samples were begun a few hours before sunset (arrow) and ended a few hours after sunset and onset of complete darkness (From Bauer, 2023 after Bauer, 19185a,b) |
 |
 |
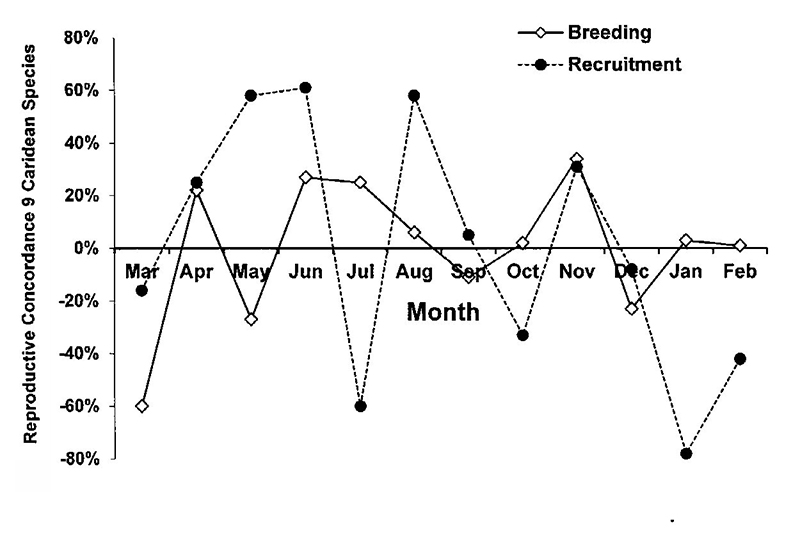
| To test hypotheses about the importance of phylogenetic vs environmental factors in breeding among species, I ranked monthly measures of (1) breeding (% ovigerous females) and (2) recruitment (% juveniles in the population) for the 9 most abundant caridean species at the Dorado P.R. seagrass beds.The Kendall concordance test (Tate & Clelland, 1957) was used to examine the null hypothesis of no joint variation among species in these measures. There was breeding all year long but the highs and lows of breeding were not particularly concordant (low joint variation) among species. On the other hand, recruitment of juveniles was highly concordant (highs and lows occurred at similar months) but there was no seasonal pattern (episodic recruitment, unknown environmental variables similarly influencing recruitment of the various species). |
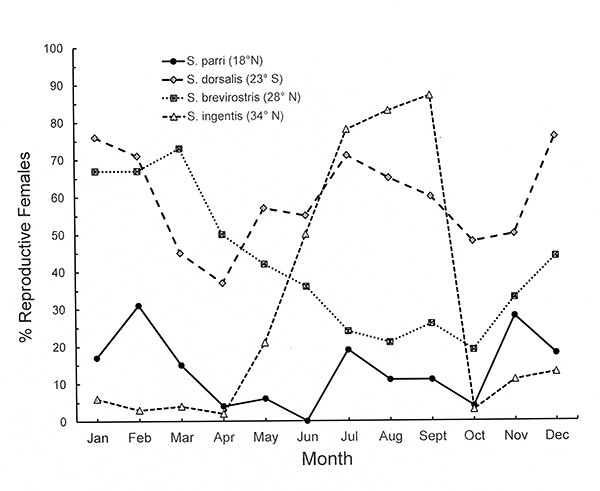
The graph shows that seasonality of reproduction in Sicyonia spp. (Penaeoidea) is correlated with latitudinal seasonality of environmental factors, continuous in the tropical species and increasingly seasonal with increasing latitude. Latitudinal variation in reproductive pattern (mature, prespawning females) is shown for four Sicyonia species (Penaeoidea): S. parri (18° N, tropical), S. dorsalis (23° S, tropical/warm temperate transition); S. brevirostris, (28° N, warm temperate) and S. ingentis (34° N, warm/cool temperate transition). Note that the S. dorsalis populations are from the southern hemisphere, where seasons are the inverse of the northern hemisphere locations. Based on data from Bauer (1992 and references therein) and (S. dorsalis ) Castilho et al. (2008). |
I have tried to relate breeding patterns to factors of
the environment which may have caused them to evolve (selective pressures).
These include the seasonality of production of larval food supplies and
the intensity of predation pressure in the environment. Although it is
sometimes hard tedious work to describe the patterns of breeding, the observations
needed to test hypotheses about factors explaining these patterns is even
more difficult. Lots of work for an interested marine biologist to do! Look also for the very good work on this theme by a group of Brazilian investigators working in southeastern Brazil (Adilson Fransozo, Maria Lucia Fransozo, Antonio Castillo (UNESP Botucatu), Rogerio Costa UNESP Bauru), Fernando Mantelatto (USP, Ribeiro Preto) and their students.
Bauer Publications
Back to Home Page