
 |
 |
Natural fouling by barnacles and other encrusting organisms on hard surfaces, e.g.. cans and bottles, in the marine environment |
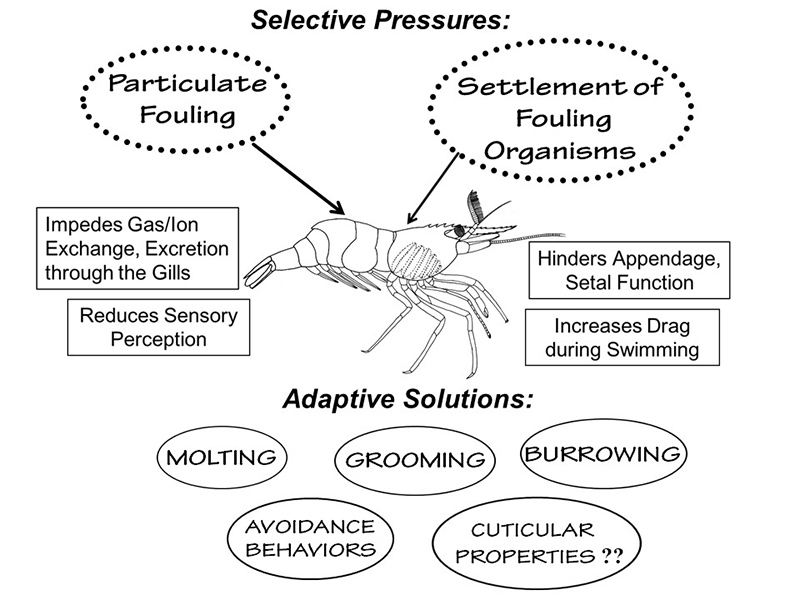
Sources of fouling and adaptive solutions for avoiding or removing such fouling in aquatic habitats (from Bauer, 2023) |
One of the most interesting types of research, in my opinion, is to determine how some structure of an organism works and how it might have evolved in the ancestor of the organisms under study. In crustaceans, this involves using a mix of anatomy, microscopy, behavioral observations, and experiments to test hypotheses about the adaptive value of a structure. Much of my work in this area has centered on "antifouling" mechanisms, i.e., structures and behaviors that help to keep a crustacean clean. Clean from what? Aquatic environments are "dirty," slightly to highly turbid with suspended particles of sediment and organic debris. There are many kinds of microbial organisms, such as bacteria, fungi, sessile protozoans, microscopic algae, as well as bigger fouling organisms, that need a solid surface to settle and grow on. The hard non-living exoskeletons of crustaceans like shrimps are such attractive surfaces. The fouling of the shrimp body by aquatic "dirt" and fouling organisms can interfere with gill function (respiration, excretion), olfaction ("sense of smell") and locomotion. In an aquatic organism, heavy fouling on its exterior would interfere with swimming just as barnacle fouling on the bottom of a ship makes its movement through the water inefficient.
Shrimps, as well as other decapod crustacean species, spend much time in grooming or brushing the body. Some appendages have brushes and combs of setae (hairs) specialized for grooming. Sensory and respiratory structures (e.g., antennae and gills) are always cleaned in some way. Many decapod crustaceans, especially caridean and stenopodidean shrimps, keep incubated embryos meticulously clean. Figured below are some illustrations of shrimps engaged in cleaning behaviors, as well as some of the structures used to clean the body.
Sensory Appendages:
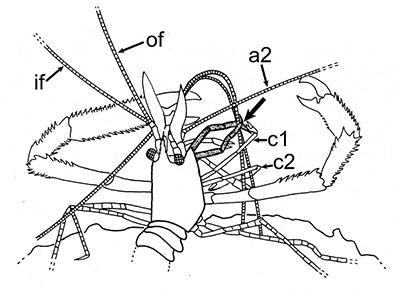
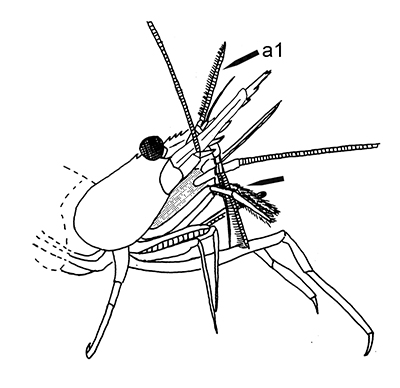
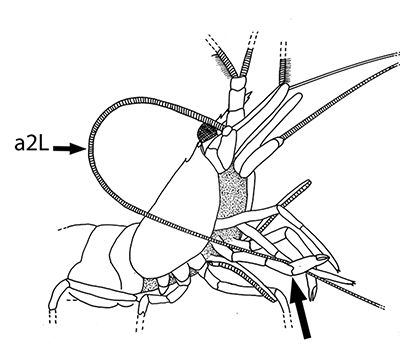
The antennules (first antennae, A1 ) are the most important chemosensory structures of shrimps and other decapods. The delicate sensory hairs on them, the aesthetascs, detect compounds dissolved in the water, even those at very low concentrations. They serve as the sense of smell (olfaction) in these crustaceans. They are cleaned primarily by brushes of multiscaled setae on the third maxillipeds. The second antennae (A2), with their long flagella, are key chemotactile (taste, touch) structures. They are cleaned by specialized brushes, the pereopod 1 carpal propodal (P1-CP) organ on the first pair of chelipeds in decapod shrimps.
 |
 |
 |
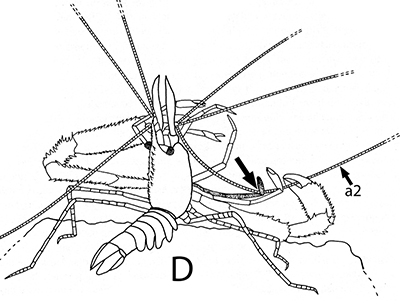 |
| A1 grooming in Stenopus hispidus by the third maxillipeds (arrow) (from Bauer, 1989) | A1 grooming in caridean Pandalus danae by third maxillipeds (from Bauer, 1975) | A2 grooming (arrow) in Pandalus danae by the propodal-carpal (P1-CP) brush on the first cheliped (note characteristic loop formed by the A2 during cleaning) (from Bauer, 1975) | A2 grooming in Stenopus hispidus by the P1-CP on the first cheliped. Note how other chelipeds help depress the antennal flagellum so that the first cheliped brushes can clean it (from Bauer, 1989) |
 |
 |
 |
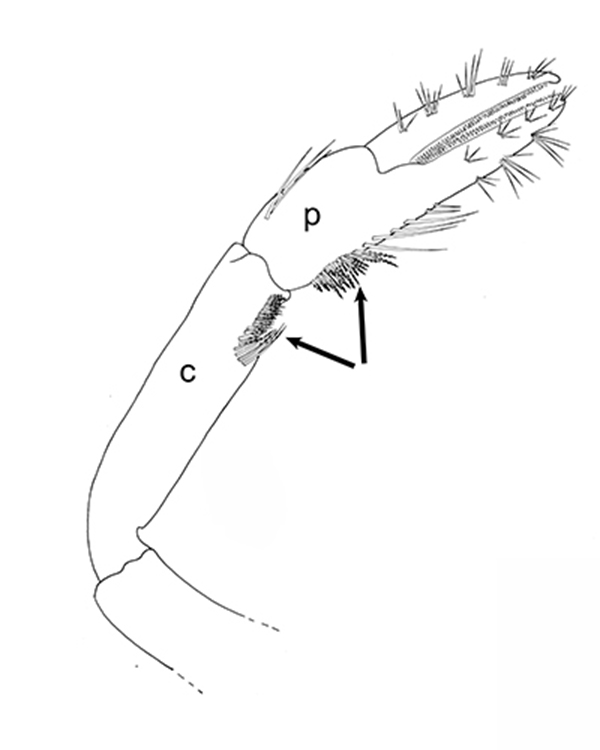
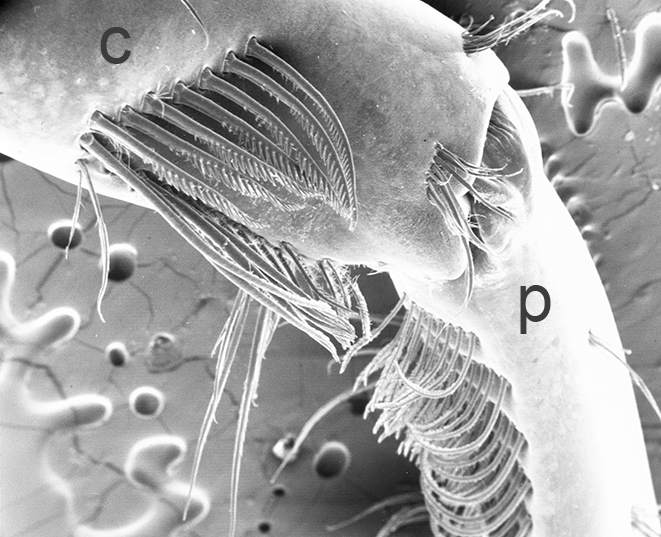
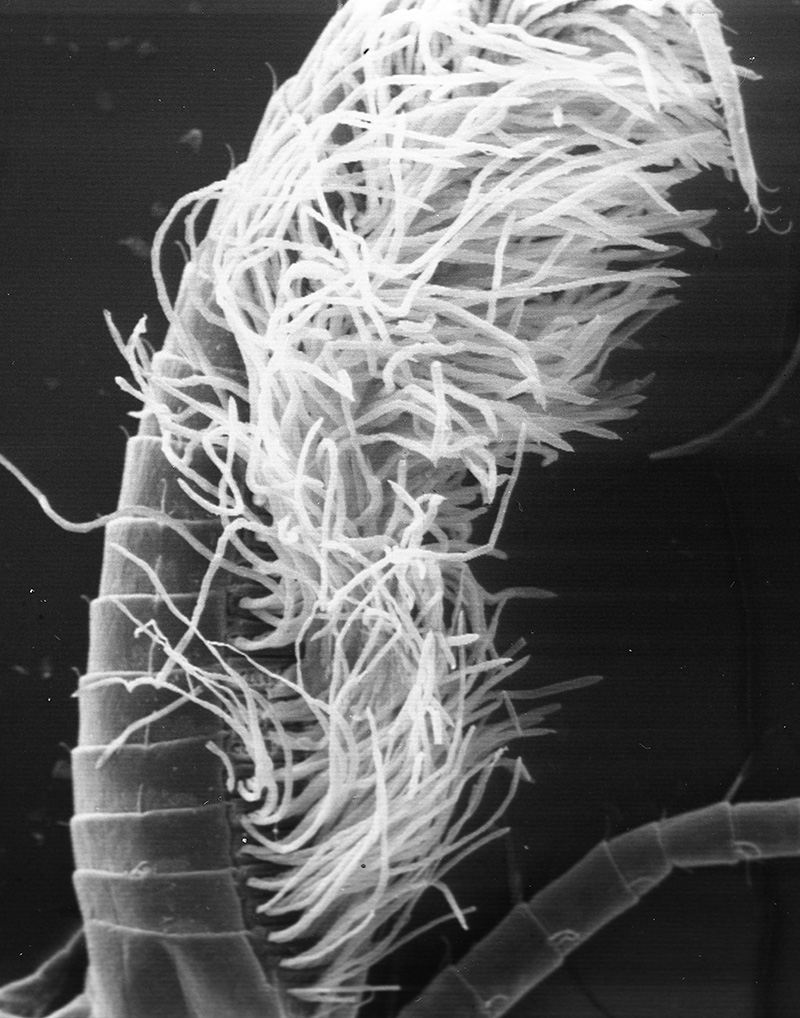
| First cheliped of the penaeid Farfantepenaeus brevirostris, showing the P1-CP antennal cleaning brushses (from Bauer, 1981) | First pereopod of caridean Pandalus danae with P1-CP cleaning brushes (c, carpus; p, produs of P1) (from Bauer, 1975) | Multidenticulate scale setules on a cleaning seta, typical of many grooming brushes (Pandalus danae) (Bauer, 1975) |
What kind of fouling can occur when antennae are not cleaned? Experiments using ablation of antennules (A1) in an experimental group of shrimps (third maxillipeds removed, appendages that groom A1) and a control group (ablation of a pair of walking legs not involved in grooming) give the answer. In one experiment, two groups were placed in perforated buckets and hung from a pier and also a lab water table supplied with a continuous supply natural) seawater. Within a few days, the antennules of experimentals began to darken noticeably. Upon examination, the aesthetascs of experimental were heavily fouled with sediment and infested with both sessile organisms, especially the filamentous bacteria Leucothrix. The chemosensory hairs (aesthetascs) became overloaded with material and broke off, depriving the shrimps of their sense of “smell,” their most important sensory input. Aesthetascs of control groups remained clean and intact. (Bauer, 1977). Similar experiments with Pandalus danae yielded similar results, although aesthetasc breakage did not occur (Bauer, 1975).
 |
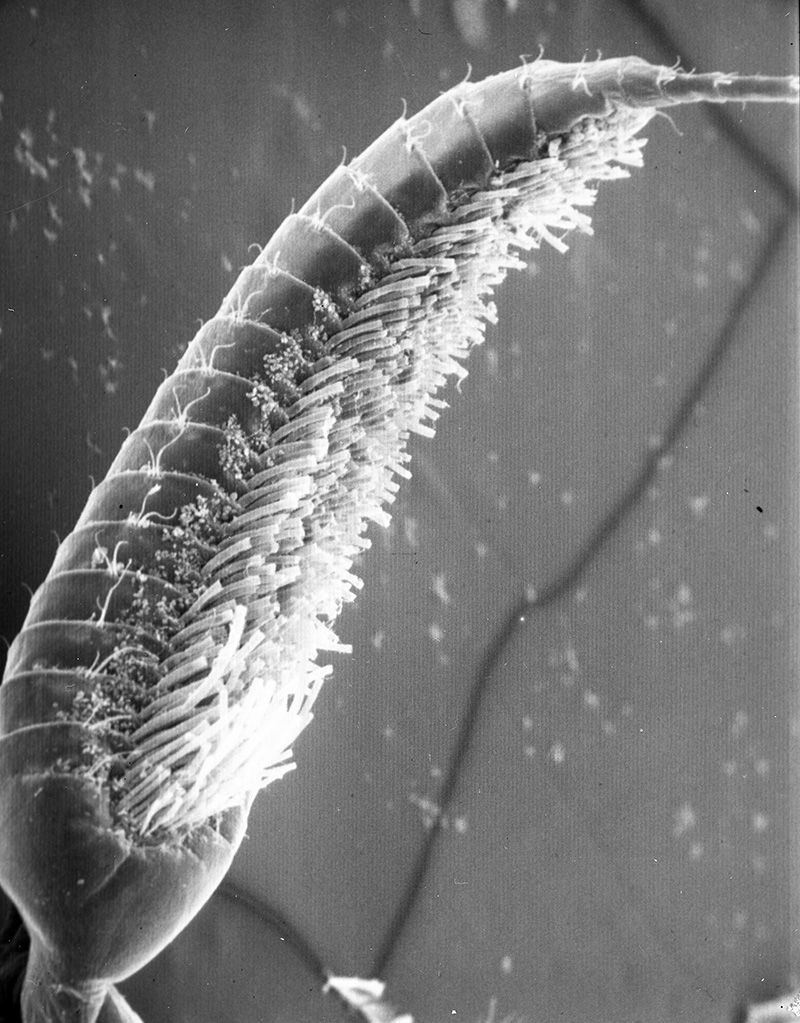 |
| Antennule of caridean Heptacarpus sitchensis from control group (antennular cleaning allowed); note the white spaghetti-looking sensory hairs (setae) termed aesthetascs. They remained clean in spite of two week exposure to natural fouling (from Bauer, 1977) | Antennule from experimental group which were exposed to same ambient fouling as controls but prevented from grooming by third maxilliped (grooming appendages) ablation. The aesthetascs became so loaded with sediment and microbial fouling organisms that they snapped off at their bases as the antennule was spun rapidly,normal behavior to circulate water among them (from Bauer, 1977) |
Gill Cleaning:
In all decapod crustaceans, including shrimps, the gills are enclosed in a branchial chamber. This confinement of gills in a narrow space protects the gills from damage and allows a rapid flow of water, powered by the beating of the “gill bailer” (scaphognathite), to pass over them. Rapid flow of water over the gills allows efficient exchange of carbon dioxide and oxygen, as well as excretion of metabolic wastes (ammonia) . However, the water entering the gill chamber, even in clear tropical waters, contains minute particulate matter (fine sediment, detritus) as well as the settling stages of sessile organism, especially microbial foulers (e.g, bacteria, protozoans). The gills must be kept clear of this fouling to function well.
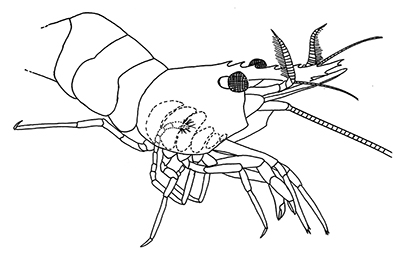
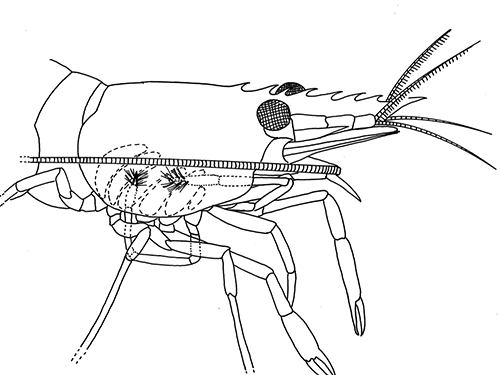
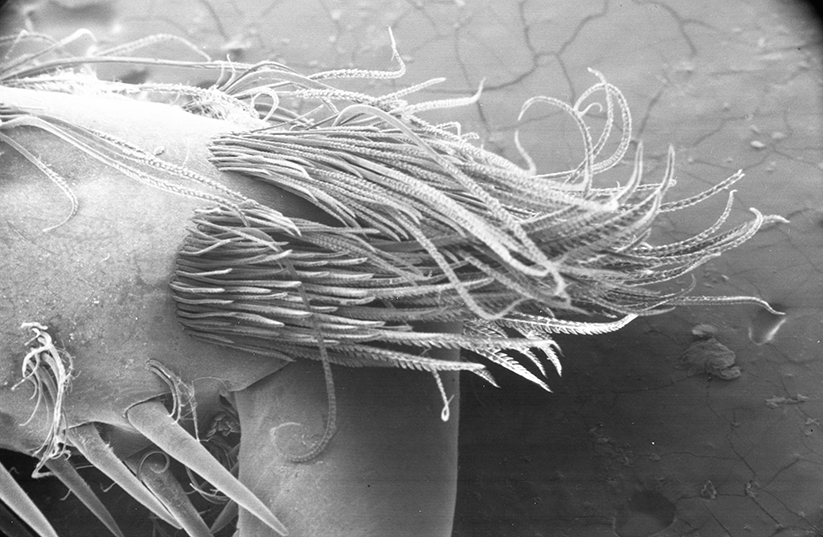
There are two basic kinds of gill cleaning, “active” and “passive. In active gill cleaning, the shrimp inserts a small first or second cheliped, depending on the taxon, into the gill chamber and brushes and picks material that has fouled the gill (see below). These grooming chelipeds are equipped with small brushes of setae (cuticular hairs) that bear an abundance of multidenticulate scales (setules) that can scrape material from the delicate gills without damaging them. This mode of cleaning occurs in stenopodidiean and many caridean shrimps, as well as anomuran crabs, but has not been observed in penaeoid shrimps, which have another type of gill cleaning mechanism (see below).
 |
 |
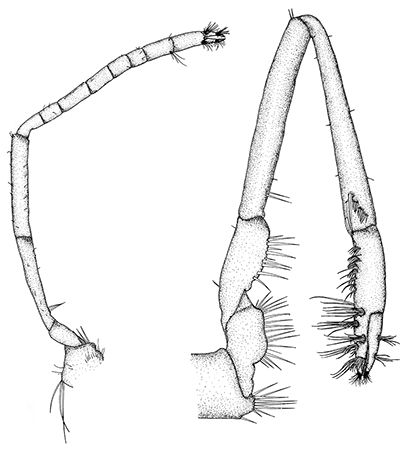 |
| Cheliped brushing of gills, each grooming cheliped inside of gill chamber of same side, caridean Heptacarpus sitchensis (from Bauer, 1979) | Cheliped brushing of gills, both grooming chelipeds inside gill chamber of right side, caridean Palaemon ritteri (from Bauer, 1979) | Left:Grooming cheliped (pereopod 2) of H. sitchensis; Right: Grooming cheliped (pereopod 1) of P. ritteri (from Bauer, 1979) |
 |
 |
| Multidenticulate setae from gill grooming brushes on cheliped 1, Palaemon ritteri (from Bauer, 1979) | Higher magnification of typical multidenticulate seta from a gill grooming structure (from Bauer, 1998) |
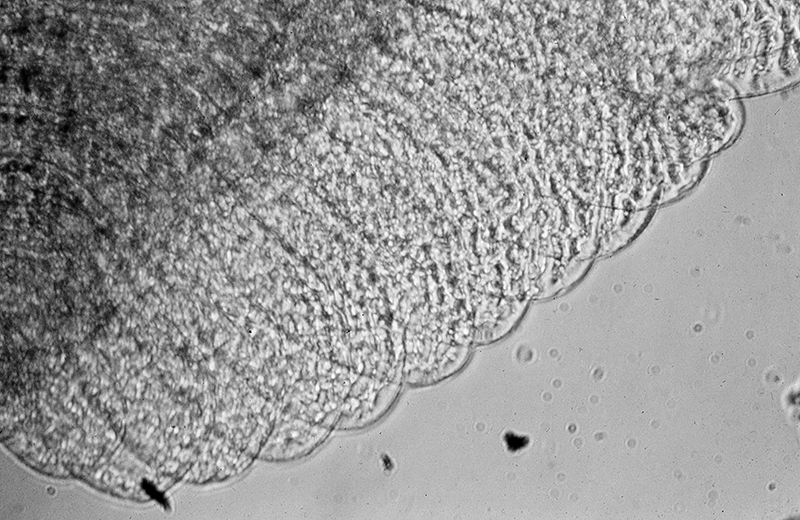
Experiments in which cleaning chelae were removed in Heptacarpus sitchensis revealed the importance of gill cleaning. As in the experiments on antennular cleaning, experimental and control groups were lowered in perforated buckets in natural seawater for two weeks or more, depending on the species. The gills within the branchial chamber became dark within a few days in experimentals but not in controls. Examination of experimental and control gills revealed heavy sediment and microbial fouling in the former, but very little if any in the latter (see below) (from Bauer, 1979)
 |
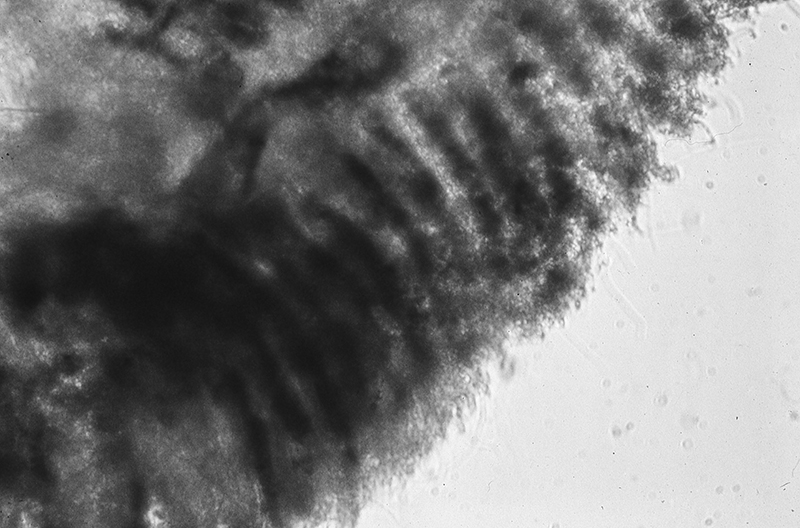 |
| Gill lamellae of control shrimp (grooming chelipeds present); no accumulation of particulate and microbial fouling after two weeks exposure to natural seawater in the field (caridean Heptacarpus sitchensis) (from Bauer 1979) | Gill lamellae of control shrimp (grooming chelipeds absent); heavy fouling by particulate and microbial fouling after two weeks exposure to natural seawater in the field (H. sitchensis) (from Bauer 1979) |
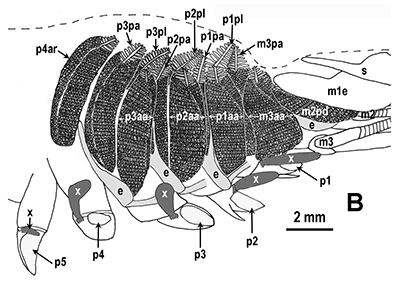
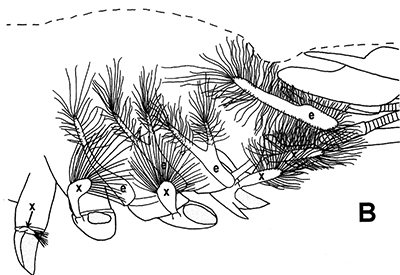
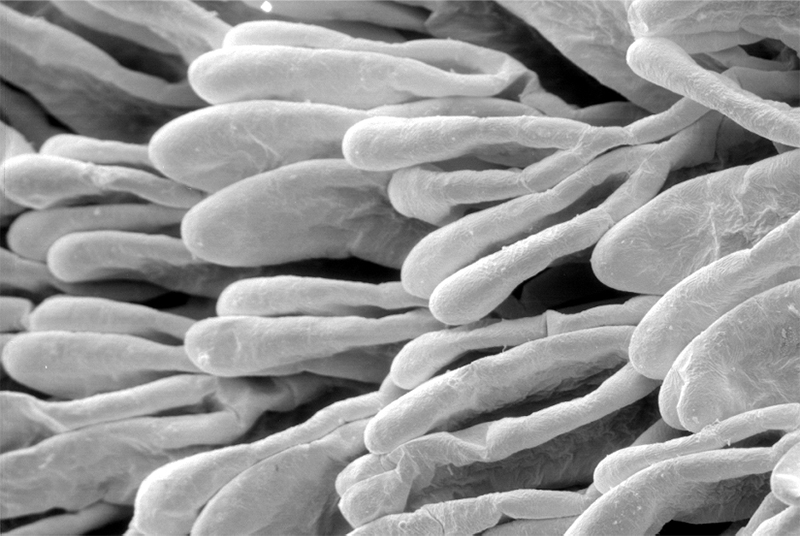
Penaeoid shrimps and some caridean species use another “passive” gill cleaning mechanism. In penaeoids, the pereopodal epipods, leaf-like delicate lamella attached to the basal coxae of walking legs and third maxillipeds, separate the gills of one appendage from those others. The epipods bear an abundance of long multidenticulate setae that wrap themselves around and among the gills. When the shrimp moves the appendages that bear these epipods, their setae jostle among and scrape the gills, keep the gills filaments free of fouling (experiments with penaeid Rimapenaeus similis, see below from Bauer, 1999).
 |
 |
 |
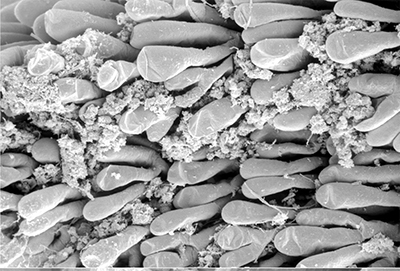 |
| Overall view of gills, epipods, exopods within gill chamber, penaeid Rimapenaeus similis (Bauer, 1999) (aa, anterior arthrobranch, ar, arthrobranch; e, epipod; x, exopod; m3, maxilliped 3, m1e, epipod of maxilliped 1; pa, posterior arthrobranch; pl, pleurobranch; p1-4, pereopods1-4; scaphognathite (gill bailer) | Distribution of gill cleaning setae on exopods (e) and epipods (x) within gill chamber, R. similis (gills removed to show exopods and epipods; only bases of pereopods and maxillipeds shown) | Clean gill filaments from passive gill cleaning experiment after two weeks of exposure to recirculating seawater table (setiferous exopods and epipods present) | Gill filaments from experimental shrimps (setiferous exopods and epipods removed) fouled with sediment after two weeks exposure to seawater in recirculating water table |
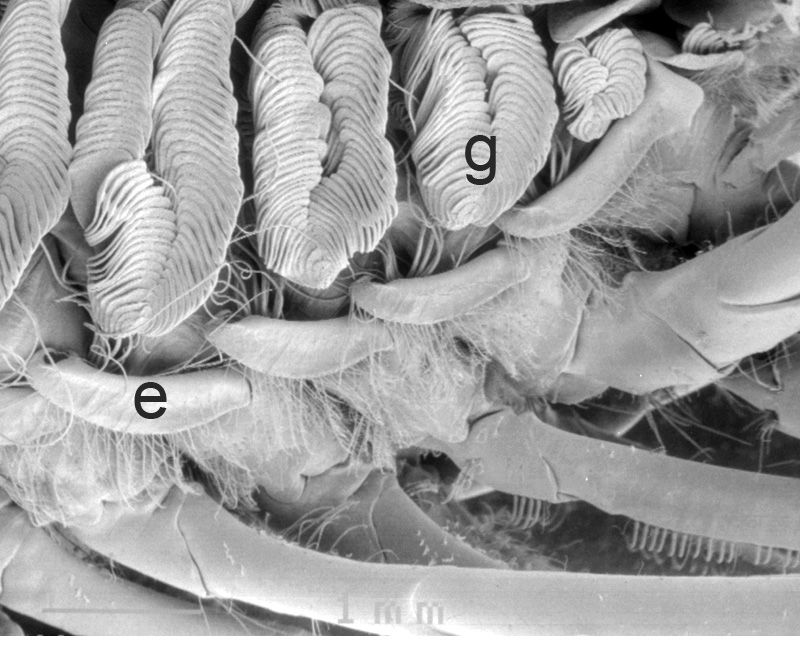
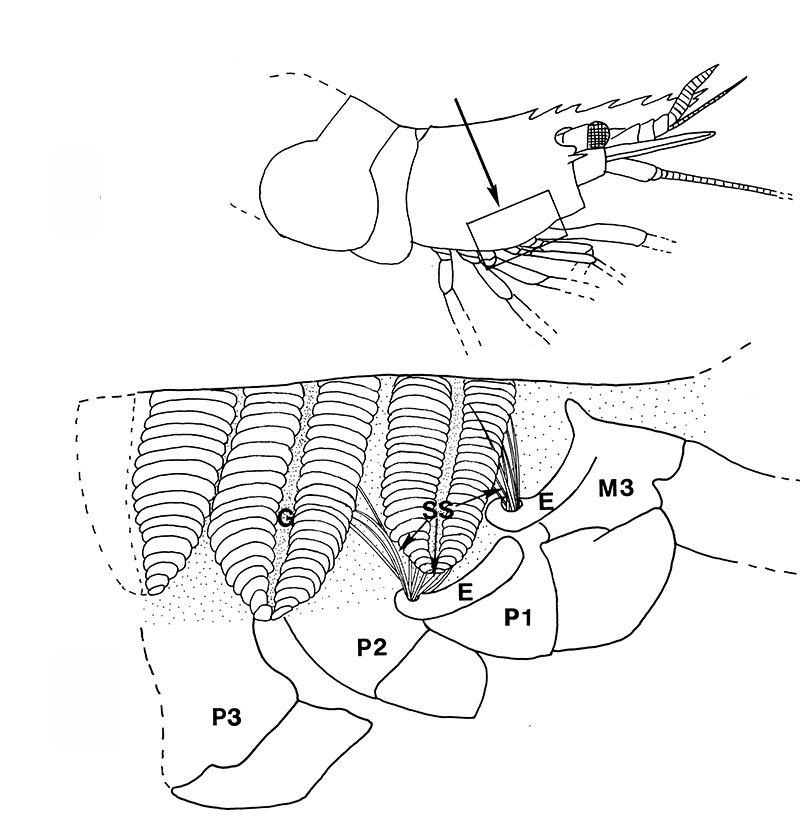
Similar experiments done on the caridean Heptacarpus sitchensis confirm this. In carideans, multidenticulate cleaning setae ("setobranchs") arise from the coxae of pereopods and third maxilliped. Hooked bar-shaped epipods on one appendage clasps around the bases of the setobranchs of the appendage just posterior help guide the setobranchs to and from the gills they clean; without them, the setobranchs become disorganized away from the gills (Bauer, 1979, figures below).
 |
 |
 |
 |
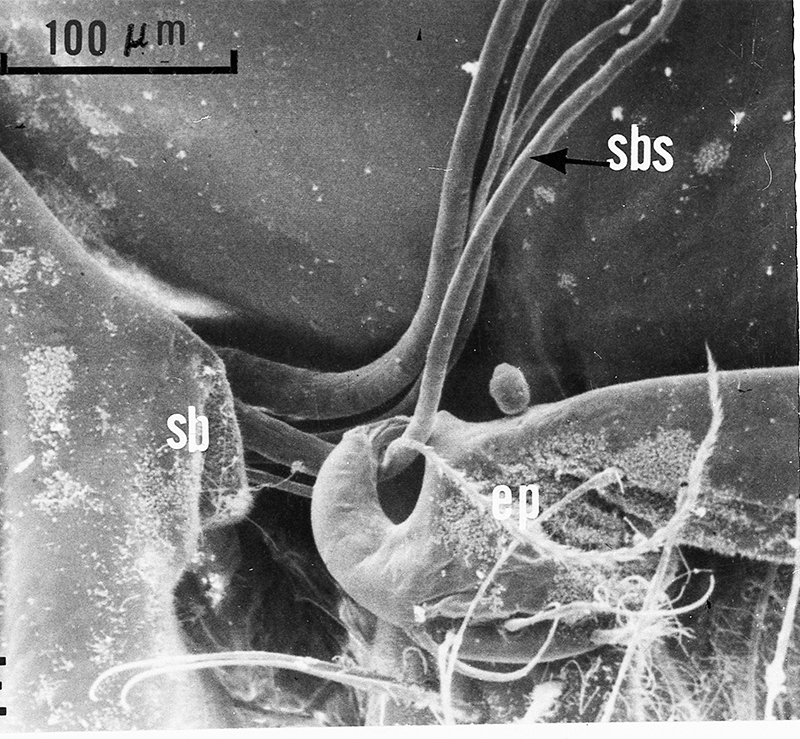
| Gills in branchial chamber of caridean Lysmata wurdemanni, showing epipods (e) hooked around setobranch cleaning setae reaching up among gill filaments | Caridean Heptacarpus sitchensis showing a portion of the gill chamher (outlined above) with gill cover removed, showing epipods (e) of Max 3 and pereopod (cheliped) 1 hooked around setobranch setae of limb just posterior | Epipod hook (ep) of caridean Betaeus macginitieae partially displaced from the setobranch setae (sbs) | Hooked epipods of various caridean species, part of the epipod-setobranch gill-cleaning system of numerous caridean species |
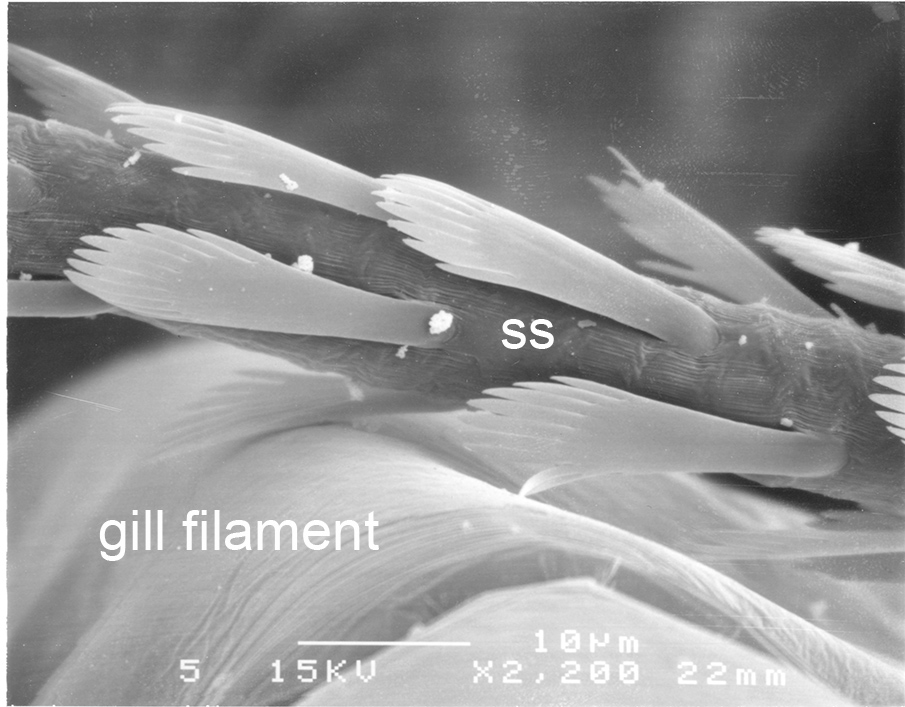
A similar system cleans the gills of crayfishes (e.g., Procambarus clarkii). In crayfishes, however, cleaning setae (setobranchs) arise from the coxae of pereopods and third maxillipeds, not from epipods as in penaeoids. In carideans that have passive cleaning, setobranchs also arise and reach up to the gills from limb coxae but they are not so numerous as in crayfishes. This has something to do with the fact that small epipods with a hook at the end gather the epipods in a bunch. When the shrimp moves these limbs, the setobranchs direct them precisely to the gills that they clean. Work on crayfish gill cleaning (Bauer, 1998, below) indicates that while setobranch cleaning of sediment is efficient (see below), it is not so efficient in cleaning of microbial fouling. If this is also true of caridean shrimp setobranch cleaning, it may explain the fact that gill brushing by chelipeds is clearly replacing setobranch cleaning in that group (Bauer, 1979).
 |
 |
 |
|
| Control gills (setobranch cleaning setae present) of still clean after 48 days exposure to fouling in a natural swamp habitat (Procambarus clarkii) (from Bauer, 1998) | Experimental gills from crayfish with setobranch cleaning setae removed) with heavy fouling after 48 days exposure to fouling in a natural swamp habitat (from Bauer, 1998) | Closeup of denticulate scale setae from a crayfish setobranch scraping over a gill filament (from Bauer, 1998) |
General Body Cleaning:
The exoskeleton of shrimps, as in other crustaceans, is composed of chitin often reinforced by calcareous compounds. It is not a living tissue, like the that of soft-bodied invertebrates. The broad surfaces of the exoskeleton of the carapace and abdomen are thus ideal for settlement of fouling organisms. Especially in shrimps, in which both forward and backward (tail flip). swimming is an essential behavior. Ships whose hulls are heavily fouled by sessile invertebrates and algae are slowed down and must expend extra fuel (energy). The fouling produces a drag on the ship impeding forward motion. Likewise, swimming shrimps encrusted with fouling organisms, both macro- and microscopic, face the same problem.
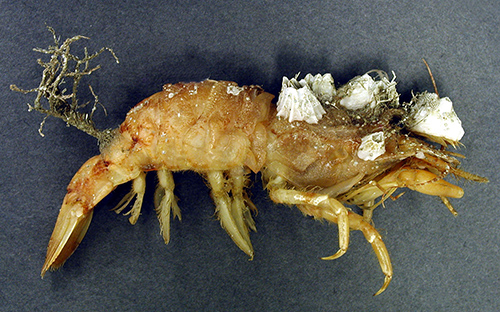 |
| Severely fouled penaeoid shrimp Sicyonia brevirostris (from Guay et al., 2011, courtesy Bernard St. Marie) |
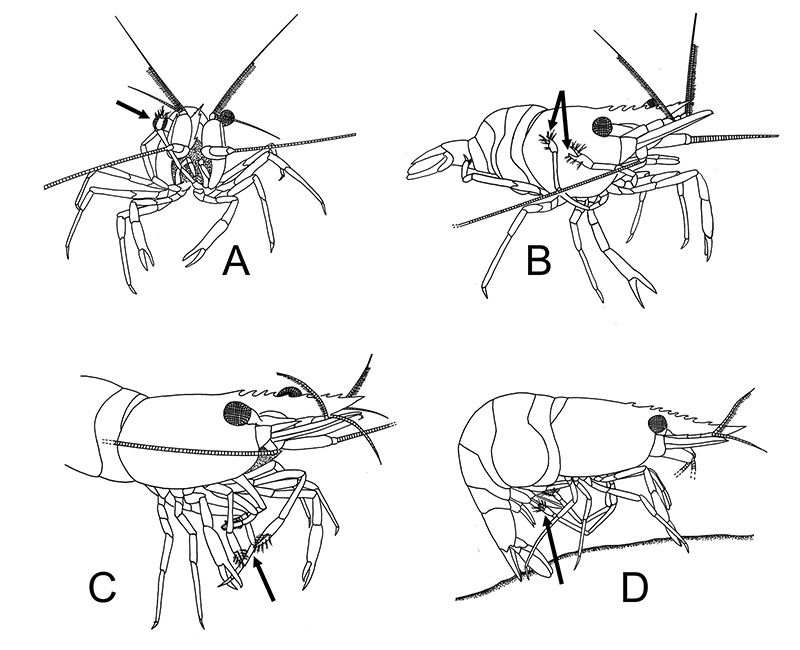
Many caridean and stenopodidean shrimps engage in general body grooming with their grooming chelipeds and posterior pairs of walking leg (pereopod 4, especially 5). Rows of serrate setae on, usually, the propodal article of pereopod 5 are used in scraping the exoskeleton of the carapace and abdomen. In atyid carideans, the dactyl looks like a comb, with a singly row of stout cleaning setae.
 |
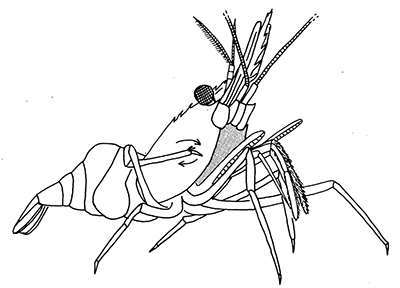 |
| General grooming with cleaning chelipeds in Palaemon ritteri of (A) an eye; (B) carapace; (C) a walking leg; (D) pleopods (from Bauer, 1979) | Pandalus danae, cleaning of carapace with a pereopod 5 grooming brush (from Bauer, 1975) |
 |
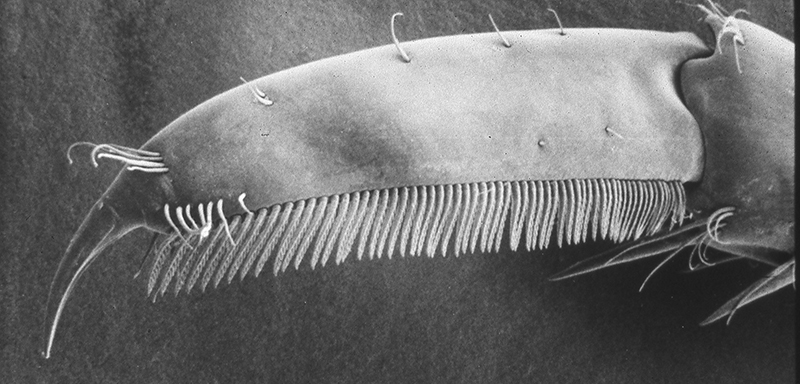 |
| General cleaning brushes on the propodus of the last walking leg (pereopod 1), Pandalus danae (from Bauer, 1975) | Dactyl of last walking leg of Xiphocaris elongata with cleaning comb (from Bauer, 1989) |
However, there are few observations on general body cleaning behavior or structures in penaeoid and sergestoid shrimps. Benthic penaeoids usually burrow just under the surface of the sand or mud sea bottoms which they inhabit, usually during the day to escape diurnal (visual) predators. Scraping of the carapace and abdomen during burrowing may make unnecessary body cleaning by appendages as in carideans and stenopodideans.
General body grooming limbs are also used by females to clean their incubated embryos. Experiments have shown that females prevented from cleaning their embryos lose many of them because of fouling. Female grooming keeps the embryos free from smothering sediment and microbial fouling, as well as infestation by small invertebrate predators, e.g. nemertean worms (Bauer, 1979)
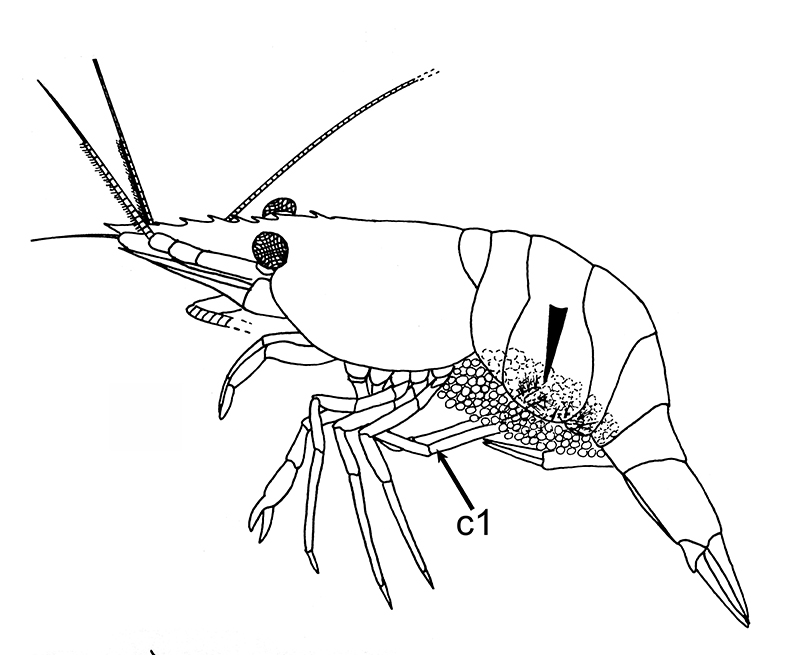 |
| Female Palaemon ritteri cleaning incubated embryos with grooming chelipeds (pereopods 1) (from Bauer, 1979) |
It is interesting to note that deep-sea stenopodidean shrimps (spongicolids) which live a sedentary symbiotic life style within hexactinellid (glass) sponges have reduced cleaning structures compared to their free-living stenopodidean relatives.
The experiments and studies above have not only revealed much about the fouling pressures acting on marine crustaceans but also about the evolutionary pathways by which different antifouling mechanisms have evolved (see Bauer, 1981,1977, 1978, 1979, 1981, 1998, 1999, 2013 and Bauer 2023, Chapter 4). However, only a handful of shrimp species have been studied in this regard, and many research opportunities remain.
Back to Home Page